
This simulation shows how neutrons and protons sit in energy levels and make up the nucleus. The Energy education team has adapted the following simulation from the University of Colorado. This process is called "enriching” uranium and is a very difficult engineering challenge. The uranium used for nuclear power in most nuclear reactors is processed to have a higher ratio of 235U to the other isotopes than normally occurs in nature.

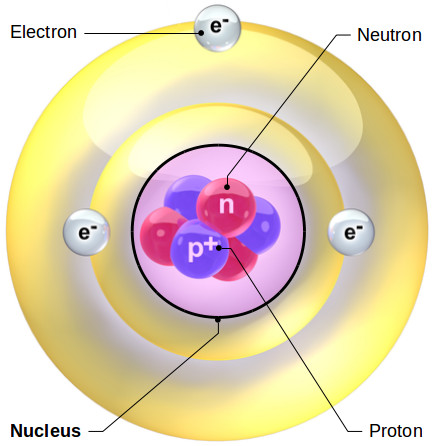
In nature, uranium exists as a mixture of 238U, 235U, and 234U. This is the only naturally occurring fissile nucleus found on Earth (although people have made other fissile isotopes of plutonium). In most types of nuclear power production, a specific isotope of uranium, 235U, is used. (See hyperphysics for more on carbon dating). 14C is famously used in archaeological radioactive dating, using its half life. 12C is the most common carbon isotope in nature. These types of atoms have 6, 7, and 8 neutrons respectively. Common isotopes of carbon include carbon-12, carbon-13, and carbon-14, often written as 12C, 13C, and 14C. Stable isotopes do not decay over time and tend to be the most commonly found isotopes of any given element.Īn example to illustrate the point would be different isotopes of carbon. Some unstable isotopes have very short half lives (less than a nanosecond), while others have extremely long ones (trillions of years). Unstable isotopes are the ones which undergo radioactive decay and in the process, change into other elements. Isotopes can be either stable or unstable. This slight difference is more pronounced in hydrogen than in heavier elements. For example, the boiling point of water with deuterium (100.7☌) is slightly higher than the boiling point of water with just protonium (100☌). There are some very slight chemical differences as a result of these differences. Note that all three of these forms of hydrogen have only one proton that's what makes it hydrogen. The radioactive form of hydrogen which is found in nature has one proton and two neutrons and is called tritium. The next most common form, with one proton and one neutron, is called deuterium. The most common of these isotopes has a proton and no neutrons and is called protonium. There are three isotopes of hydrogen found in nature. As an example, hydrogen has only one proton. This makes chemically separating isotopes very difficult. Instead, different isotopes have different mass and have different tendencies to radioactively decay, or change over time.ĭifferent isotopes have very similar chemical properties because chemistry is determined by the electric charge. Having different numbers of neutrons in the nucleus will not affect the charge of the atom. Atoms of the same element all have the same number of protons, by definition, because elements are defined by how many protons they have. Each proton has a charge of +1, each electron a charge of -1, the neutrons have no charge (neutral charge-neutron). Atoms have protons and neutrons in a dense nucleus at the centre, and electrons in orbitals around the nucleus. Īll matter is made up of atoms in various arrangements. Changing the number of neutrons in an atom changes that atom's atomic mass. Isotopes of an element are atoms with the same number of protons, but a different number of neutrons. In other words, the proton number determines the element. The number of protons in the nucleus of an atom establishes how the atom will react chemically. Nuclei of carbon's three most common isotopes.


 0 kommentar(er)
0 kommentar(er)
